Highest possible temperature.
In this article we will discuss the question "What is the highest temperature possible?" You might already know what is the lowest possible temperature. It is 0 degrees per Kelvin, or "absolute zero" as physic engineers usually call it. If looking at molecular level, than temperature is nothing more than molecule movement speed. In gases and liquids molecules or atoms are in constant movement. The higher temperature substance has, the faster molecules move in it.
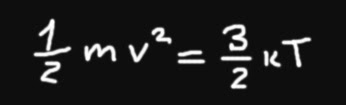

When any substance temperature goes down to absolute zero, it means, that all molecules in substance do not move in respect to each other. In europe we use Celsius temperature scale, so when converting 0 degrees Kelvin to celsius it is -273.15C. Allrighty, that is freaking cold.
Now lets try to figure out - what is the maximum temperature possible.
It turns out that this question needs more constraints applied to it before question can be answered. First we will assume that molecules in substance are moving with almost speed of light (299 792 458 m / s). That is because the speed of light is maximum available speed in our universe. Nothing can move faster than that. Also we will need to choose particular chemical element that we will use, because temperature is proportional to atom mass. The heavier element, the highest temperatures it can reach. It means, for example, that if hydrogen (H) atoms, that are very light, move at 300 m/s, it will have lower temperature than Mercury (Hg) atoms moving in the same speed, because Hg atoms are much heavier. Ok, here is the formula we will use to calculate the maximum possible temperature. This formula ties together molecular speed with temperature.
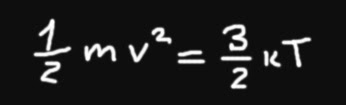
Used values:
m - one molecule mass
v - avarage molecule speed
k - Boltzmans constant
T - temperature
I looked in the periodic table, and finally decided to choose element lead (Pb), because it is last stable element in periodic table. Everything heavier than Pb, is radioactive and will decay with the time. So, let it be lead. The molmass for lead is 207.2 grams per mole. For those who have forgot what molmass is - just a quick note. Molmass is the mass expressed in weight units, such as grams or kilograms for 6.0224179×1023 molecules.
Lets do some calculations now:

So the final answer is that maximum possible temperature for Lead is approx 75 milion degrees Kelvin. And that is quit plausable. Because temperature at sun, that consists of Hydrogen and Helium, core is 15 milion degrees.
This result of course is theoretical, because if there were lead with such a high temperature, atoms would simply collide and form different elements right away. Also I need to admit, that lead in such a high temperature would be in plasma state, which means, that there would not be regular Lead atoms, bet lead nucleus with bunch of electrons floating around.
The information written above can not be used in any scientific document, or referenced in any work that requires respectable sources. I do not possess any degree of physics and these calculations I do just for fun.

Comments
or in a textbook for students ?